Perché molti farmaci testati sugli animali falliscono negli studi clinici?

Gli studi clinici garantiscono che i pazienti umani e animali ricevano medicinali più sicuri possibile. Nessun farmaco può essere sicuro al 100%, cioè non avere alcun effetto collaterale. Questo è il motivo per cui i test sugli animali e sugli esseri umani sono così rigorosi, in modo da garantire lo sviluppo di farmaci che siano il più efficacie possibile e con il minor numero possibile di effetti collaterali.
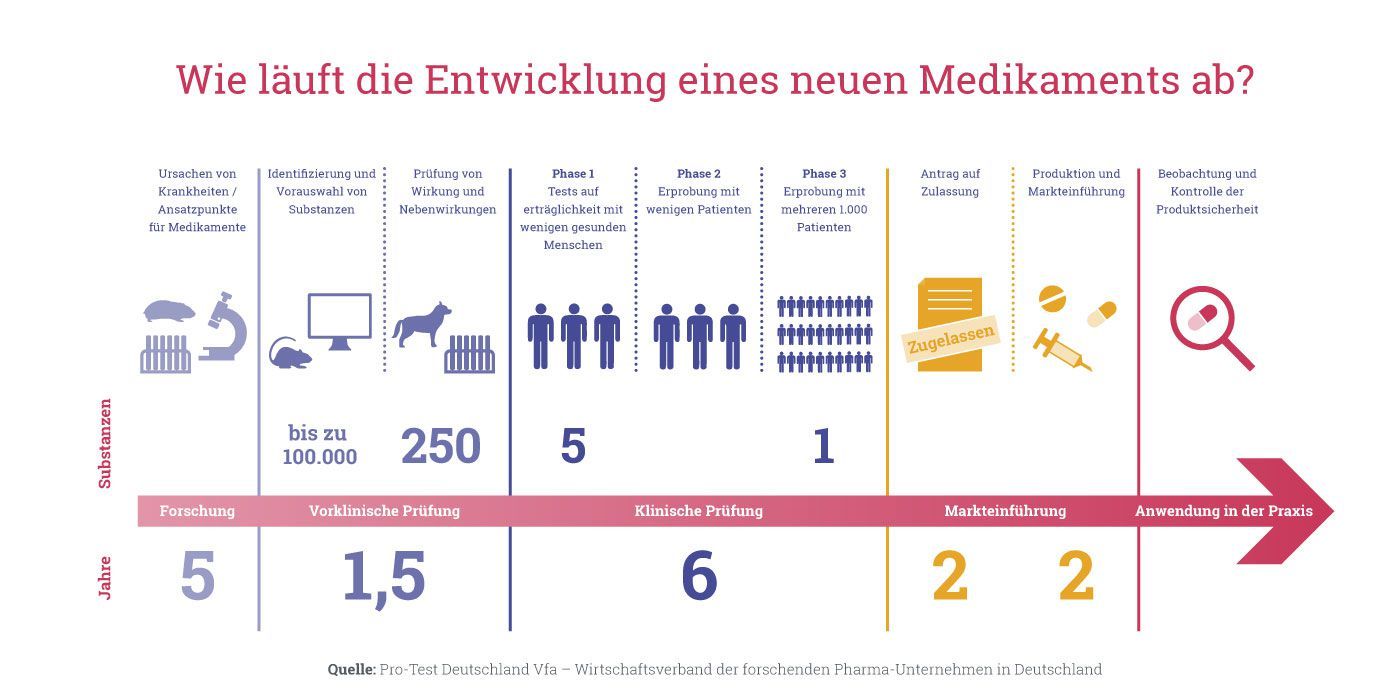
Per fare questo, bisogna capire come funzionano questi test farmacologici: Affinché un nuovo composto venga approvato come farmaco e immesso sul mercato, deve passare attraverso diverse fasi di test. Inizialmente, vengono effettuati solo studi su colture cellulari e animali da laboratorio. Se questi test hanno successo, i nuovi farmaci vengono prima testati su poche persone e - se il beneficio è considerato grande e il rischio basso - gradualmente su un numero sempre maggiore di persone.
Circa un terzo dei farmaci sperimentali non supera i test di sicurezza preclinici (cioè gli studi sugli animali) perché si rivelano inefficaci o troppo pericolosi. Molte sostanze falliscono poi falliscono nelle fasi successive degli studi clinici per vari motivi. Il motivo per cui un preparato non arriva sul mercato è spesso è spesso perché test su colture cellulari o esperimenti su animali lo hanno classificato come troppo pericoloso per l'uomo. Questo significa la fine per l'ulteriore sviluppo di una preparazione.
Queste fasi normative sono descritte in dettaglio qui; l'ulteriore sviluppo di un farmaco dipende dal completamento con successo di ogni fase:
Fase preclinica
Le colture di cellule o di tessuti vengono utilizzate per studiare il funzionamento di una sostanza. Se ci sono indicazioni affidabili che questa sostanza è teoricamente efficace in un organismo, si effettuano esperimenti sugli animali. Inizialmente, si inizia con piccoli studi con pochissimi animali. Le domande a cui viene data risposta sono: "C'è un possibile beneficio terapeutico?", "Il composto è sufficientemente sicuro per essere utilizzato nell'uomo nella fase I?". Inoltre, nei test sugli animali vengono condotti studi sulla dose, l'effetto e la distribuzione nell'organismo (farmacologia e farmacocinetica). Nel successivo sviluppo preclinico, vengono poi effettuati anche ulteriori test sugli animali per testare la compatibilità (test di tossicità), per esempio, per escludere malformazioni nel fetio La maggior parte di questi studi preclinici su animali sono regolamentati in termini di dimensioni e domande di ricerca dai requisiti normativi di Swissmedic o dell'agenzia americana American Food and Drug Agency (FDA). I risultati vengono esaminati dalle agenzie di regolamentazione, e gli studi clinici sull'uomo sono consentiti solo con l'approvazione di queste autorità. Un'azienda farmaceutica deve quindi effettuare test sugli animali se vuole immettere sul mercato un farmaco, perché altrimenti non le sarebbe consentito effettuare test clinici sulla sostanza nell'uomo.
Fase I
Questo è il primo studio nell’uomo. Questa fase può essere iniziata solo se i risultati dei precedenti test sugli animali sono stati positivi e la sostanza è considerata sufficientemente sicura da osare un primo test sull’uomo. Solo poche persone vengono testate in condizioni rigorose e sotto stretto monitoraggio per determinare se un principio attivo ha successo nel trattamento o se è sicuro anche per ll'uomo. Questa tollerabilità viene solitamnente esaminata su sei-dieci volontari sani o in pazienti gravemente malati per i quali non sono disponibili altre opzioni terapeutiche. Le domande a cui i ricercatori vogliono rispondere nella fase I sono: "Quali sono gli effetti del farmaco negli esseri umani?", "Quali effetti collaterali si verificano?", "Il farmaco è abbastanza sicuro da essere utilizzato in in un gruppo più ampio di pazienti?".
Fase II
Se nella fase I sono state trovate sufficienti indicazioni che un farmaco è benefico e non sono emersi problemi di sicurezza, il principio attivo può essere ulteriormente studiato nella fase clinica II. Ora viene testato su un piccolo numero di pazienti selezionati. A seconda del tipo di malattia contro la quale viene utilizzato il principio attivo, viene condotto uno studio di fase II su venti- trecento persone. Il focus qui è su domande come: " In che modo il successo del trattamento dipende dal dosaggio?" oppure "Qual è il regime terapeutico più efficace?".
Anche in questa fase, ci sono ancora studi paralleli sugli animali per testare l'effetto del farmaco sulla riproduzione.
Fase III
Se i risultati delle fasi I e II sono incoraggianti, inizia la fase clinica III, in cui un nuovo farmaco viene studiato ancora più da vicino in un ampio gruppo di pazienti in condizioni reali. Qui viene testato da trecento a tremila o anche più persone. La fase III, l’obiettivo principale èi chiarire: "La sicurezza e l'efficacia del nuovo farmaco sperimentale sono migliori, peggiori o uguali a quelle di trattamenti consolidati comparabili?", "Come si comporta il principio attivo studiato in combinazione con altri farmaci?" oppure " Qual è il modo migliore per prescrivere il farmaco ai pazienti?".
Nella fase III, gli esperimenti sugli animali sono ancora in corso in parallelo per escludere il più possibile un effetto cancerogeno dopo un uso a lungo termine.
Fase IV
Dopo il completamento con successo della fase III, il farmaco può essere approvato, ma continuerà ad essere monitorato negli studi clinici della fase IV registrando e valutando gli effetti collaterali. Nella pratica medica quotidiana, un numero molto elevato di pazienti assume il farmaco, in modo da poter ottenere molti dati sulla sua efficacia e sugli effetti a lungo termine. Questo permette anche di identificare effetti collaterali molto rari.